In the wonderful world of chemistry, the discovery of new substances is often accompanied by surprises and surprises, and the discovery of 18-crown-6 CAS 17455-13-9 is no exception. In 1967, when chemist Charles J. Pedersen was conducting a routine organic synthesis experiment, he originally studied a linear polyether compound, but accidentally obtained a compound with a ring structure, and after further research, 18-crown-6 came into people’s vision. At the time, the chemical community was very new to this compound with a special ring structure and multiple oxygen atoms. Pedersen dug deeper and found that 18-crown-6 had many unusual properties, such as its ability to form stable complexes with metal ions, which was a new discovery in chemistry at the time. It made scientists realize that in addition to traditional chemical bond interactions, there are special interactions with ions through large ring structures, which opened the door to crown ether chemistry and laid the foundation for the development of subsequent supramolecular chemistry.
18-crown-6, the name indicates the uniqueness of its structure. “18” represents the total number of atoms in the ring, and “6” represents the number of oxygen atoms in it. It consists of 12 carbon atoms and six oxygen atoms holding hands in a ring structure, like a delicate molecular bracelet, this special structure is very rare in the field of chemistry. In this ring, oxygen atoms, by virtue of their electronegativity, make the entire molecule with a certain polarity, while carbon atoms form the basic skeleton of the molecule, holding up the entire structure. It is this ring structure of carbon and oxygen atoms that gives 18-crown-6 CAS 17455-13-9 its unique properties, enabling it to form stable complexes with metal ions. It usually occurs as a white crystal at room temperature. Its melting point is between 42 and 45°C, which is not so hot that a little heating can transform it from a solid to a liquid. The boiling point is 116 ° C, and under certain pressure conditions, it gradually turns into a gas. It is also soluble in water and can interact with water molecules to form a uniform mixing system.
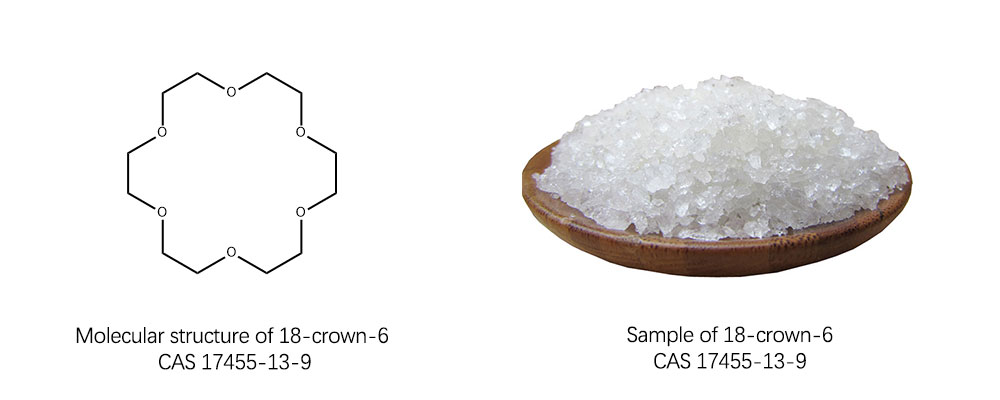
With its unique molecular structure, 18-crown-6 CAS 17455-13-9 has shown excellent application value in many fields, and has become one of the key materials to promote technological progress in various fields, providing innovative solutions to solve practical problems.
Energy field
In the energy sector. Take lithium-ion batteries, which can be described as the heart of electric vehicles and energy storage systems, and 18-crown-6 is like an injection of vitality to this heart. In the electrolyte design of lithium-ion batteries, it plays an important role by virtue of its excellent thermal stability and selective complexation of lithium ions. When the battery is in a high temperature environment, ordinary electrolyte molecules are more active, resulting in a decline in battery performance, and 18-crown-6 CAS 17455-13-9 can be closely combined with lithium ions to form a stable complex, which not only improves the cycle stability of the battery under high temperature conditions, but also greatly enhances the safety. It provides a solid guarantee for the stable operation of electric vehicles in high temperature environment and the reliable work of energy storage system.

Materials science field
When combined with polymer materials, 18-crown-6 CAS 17455-13-9 can give the material intelligent responsiveness, so that the material can respond to changes in the external environment, such as temperature, pH and other environmental factors change, the performance of the material can be automatically adjusted; It can also give the material self-healing ability, when the material is slightly damaged, it can automatically repair, extending the service life. These new functions and features have injected new vitality into the development of high-tech fields such as aerospace and electronic information, and helped these fields continue to achieve technological breakthroughs.
Chemical analysis and testing
In the field of chemical analysis and detection, 18-crown-6 can accurately identify various metal ions. Its working principle is based on the specific complexation reaction with metal ions. Scientists have designed highly sensitive and highly selective ion sensors using 18-crown-6 CAS 17455-13-9, which can monitor and measure the concentration changes of specific metal ions in real time, and play an important role in environmental monitoring, medical diagnosis and other fields.

With the rapid development of science and technology, new energy vehicles, energy storage systems, aerospace and other emerging industries have mushroomed, bringing unprecedented opportunities for the development of 18-crown-6. Unilong offers an efficient, low-cost, high-purity production method in the preparation of 18-crown-6. We are a professional supplier of chemical raw materials, quality assurance, fast delivery, off-the-shelf supply.