What is potassium myristate CAS 13429-27-1?
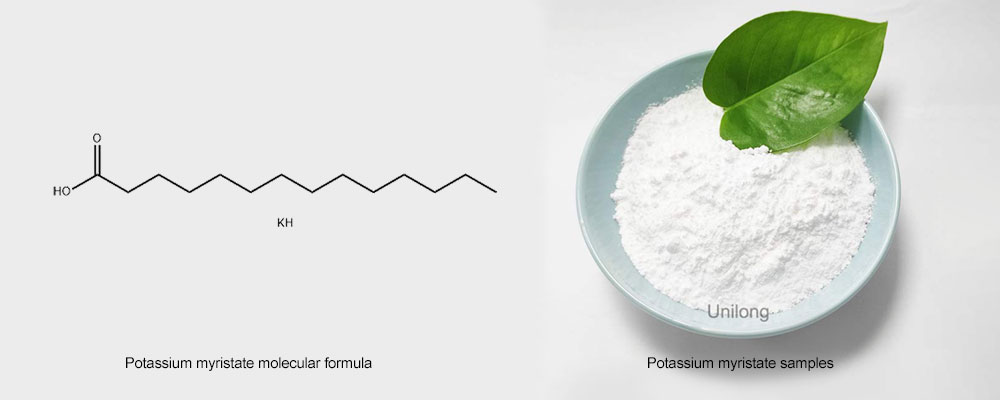
Potassium myristate, alias potassiumtetradecanoate, CAS number is 13429-27-1, molecular formula is C14H29KO2, molecular weight reached 268.48. From the point of view of chemical structure, it is an organic salt produced by the reaction of myristic acid and potassium hydroxide. Myristic acid is a saturated fatty acid commonly found in vegetable oils such as coconut oil and palm oil, as well as some animal fats. When myristic acid and potassium hydroxide meet in an acid-base neutralization reaction, the carboxyl group (-COOH) in myristic acid combines with the hydroxide group (OH) in potassium hydroxide to form water, while potassium ion (K +) combines with the myristic ion (C14H27COO⁻) to form potassium myristic acid.
Potassium myristate CAS 13429-27-1 at room temperature and pressure is usually presented as a white to light yellow powder substance, delicate texture, giving a relatively dry intuitive feeling. It has certain solubility characteristics, can show good solubility in water, and can be evenly dispersed in water to form a solution, which makes it play a good role in some water-based system products. However, when it encounters organic solvents, such as common ethanol, ether, etc., the solubility will be greatly reduced and almost insoluble. This is because the molecular structure of potassium myristate determines that it is more inclined to interact with water molecules, and the interaction between organic solvent molecules is weak.
From the point of view of chemical properties, potassium myristate is a strong base and weak acid salt. When it is dissolved in water, a hydrolysis reaction occurs. The myristate ion (C14H27COO⁻) binds to hydrogen ions emitted by hydro electricity (H +). This causes a relative increase in the concentration of the hydroxide ion (OH⁻) in solution which causes the alkaline appearance of the solution. Under general storage conditions, potassium myristate has good stability and can keep its chemical structure and properties unchanged for a long time. However, if it is exposed to high temperature, high humidity or strong acid and alkali environment, its stability will be challenged. The decomposition reaction of potassium myristate may occur in high temperature environment, resulting in the decrease of its active component content. Under strong acid-base conditions, it will chemically react with acid or base to form other substances, thus losing its original properties.
Potassium myristate plays an important role in cleaning
On the big stage of cleaning products, potassium myristate CAS 13429-27-1 plays a key cleaning role. Its cleaning principle is based on its unique chemical structure and surface active properties. The potassium myristate molecule consists of a hydrophilic head (the carboxylate part) and a hydrophobic tail (the long-chain fatty acid part), a special structure that allows it to form micelles in water. When potassium myristicate comes into contact with dirt and oil on the skin surface, its hydrophobic tail is inserted into the oil molecules, while the hydrophilic head is oriented towards the water phase. In this way, potassium myristate can wrap the oil, forming tiny micelles, which can be evenly dispersed in the water and washed away with the water flow, so as to achieve a clean effect.
Potassium myristate is widely used in facial cleansers. Potassium myristate works in concert with other soap-based ingredients to penetrate deep into pores and strongly cleanse the skin of oil, dirt and aged keratin. For people with oily skin, use a facial cleanser containing potassium myristate, which can effectively remove excessive oil from the face and keep the skin fresh and clean. However, due to its strong cleaning power, it may be irritating for people with dry and sensitive skin, and the frequency of use should not be too high.
In body wash products, potassium myristate can quickly combine with dirt, sweat and oil on the surface of the body, by producing a rich foam, these dirty things away from the skin, so that we can feel fresh and clean skin after bathing. At the same time, the shower gel also adds some spice ingredients, while cleaning, to bring users a pleasant fragrance experience.

While potassium myristate is excellent at cleansing, it is not “friendly” to all skin types. For dry skin, due to less skin oil secretion, the moisturizing ability of the skin is relatively weak, and the water content of the stratum corneum is low. While the strong cleaning power of potassium mystate removes the dirt and oil on the skin surface, it will excessively clean the oil secreted by the skin itself, causing the skin’s moisturizing barrier to be damaged to a certain extent, resulting in more dry skin and water shortage, tightness, peeling and other uncomfortable symptoms. For example, some people with dry skin who use a facial cleanser containing potassium myristate can feel as if their face is wrapped in a tight film, and in severe cases, there will be fine dandruff.
The epidermal layer of sensitive skin is thin, the barrier function of the skin is fragile, and the resistance to external stimulation is poor. The alkaline properties of potassium myristate may upset the original acid-base balance on the skin surface, stimulate nerve endings in the skin, and trigger allergic reactions in the skin. For people with sensitive skin, after using cleaning products containing potassium myristate, the skin may quickly appear redness, itching, tingling and other symptoms, seriously affecting the health and comfort of the skin.
If after the use of products containing potassium myristate, the skin appears redness, itching, stinging and other uncomfortable symptoms, this is likely to be the skin has an adverse reaction to the product, at this time should immediately stop using. At the same time, do not scratch the skin with your hands at will, so as not to increase the risk of skin damage and infection. You can gently rinse the skin with water to remove the residual product components on the skin, and then observe the skin condition. If the symptoms continue to not relieve or worsen, be sure to go to the hospital dermatology treatment in time, let a professional doctor for diagnosis and treatment.
When choosing products containing potassium myristate, be sure to maintain a rational and scientific attitude. Know your skin and make decisions based on your needs. If you have oily skin, moderate use of cleansing products containing potassium myristate can help you effectively cleanse your skin and keep it fresh. But if you have dry or sensitive skin, you need to choose carefully or look for other, gentler cleansing ingredients instead. At the same time, in the process of use, strictly follow the use recommendations and precautions to avoid damage to the skin due to improper use. I hope everyone can keep the skin healthy and beautiful through scientific choice and correct use.